Abstract
Despite novel therapies, 5-7% of chronic lymphocytic leukemia (CLL) patients experience transformation into a high-grade lymphoma, most commonly diffuse large B-cell lymphoma. This condition, known as Richter's syndrome (RS), is characterized by an aggressive clinical course and universally poor prognosis, with a median survival of 5 to 14 months.
Intrinsic apoptosis is a cell death program triggered by stress stimuli and regulated at the outer mitochondrial membrane (OMM) by the Bcl-2 protein family, which includes the pro-apoptotic effectors Bax and Bak, the BH3-only pro-apoptotics Bim, Bid and Puma, and the anti-apoptotic relatives Bcl-2, Bcl-xL, Mcl-1, and Bfl-1. Overall, the anti-apoptotic proteins sequester the pro-apoptotic members thus preventing the oligomerization of the effectors and the permeabilization of the OMM. CLL cells are remarkably close to the apoptotic threshold (i.e., they have high apoptotic priming), and are highly dependent on the anti-apoptotic protein Bcl-2 for survival. Given the paucity of RS pre-clinical models and the rarity of this disease, no study has been conducted about the evolution of apoptotic priming and anti-apoptotic dependencies during Richter's transformation.
In this work, we used intracellular BH3 profiling (iBH3) to perform a comparative analysis of the apoptotic priming and anti-apoptotic dependencies of CLL and RS cells. CLL cells were withdrawn from peripheral blood of 17 patients (N=17, 9 treatment-naïve and 8 relapsed/refractory). The RS cohort (N=8) consisted of 4 recently established patient-derived xenografts (PDX: RS9737, RS1050, IP867, RS1316), 1 RS cell line (U-RT1), and 3 primary samples (RSVR1, RSVR2, RSCNO) collected from patients with RS in leukemic phase. Samples from 2 patients were longitudinally collected either before and after high-grade transformation.
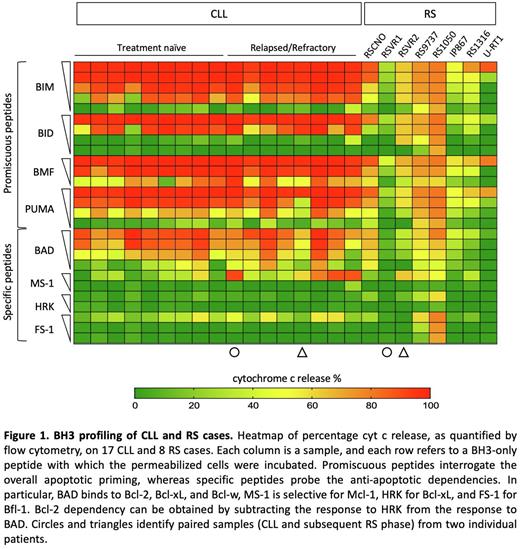
We found that RS cells released inferior amount of cytochrome c (cyt c) than CLL cells upon stimulation with promiscuous peptides, indicating decreased apoptotic priming (Fig. 1). The mean 90% effective maximal concentration of Bim, a biomarker of chemosensitivity, was 0.10±0.09 μM for CLL and not reached for RS cases. Importantly, decreased apoptotic priming was evident in RS but not in relapsed CLL samples, suggesting that high-grade transformation and not simply disease relapse is required to modulate the apoptotic priming. iBH3 of CLL/RS paired samples further confirmed the loss of apoptotic priming as disease transforms. To investigate the anti-apoptotic dependencies, CLL and RS cells were incubated with a panel of pro-apoptotic peptides that specifically antagonize distinct anti-apoptotic members. RS cells proved less Bcl-2 dependent than CLL cells (Fig. 1). Specifically, RS9737 and RS1050 showed co-dependencies upon Mcl-1/Bfl-1 and Mcl-1/Bfl-1/Bcl-xL, respectively. RSVR2 proved to be primarily Mcl-1 dependent, while RSCNO was the only one maintaining some degree of Bcl-2 dependency. The additional 4 RS samples did not show any specific anti-apoptotic dependency and harbored different nuances of class A anti-apoptotic block. Kinetic analysis of caspase 3/7 activation following venetoclax treatment further demonstrated decreased sensitivity to Bcl-2 antagonism of RS with respect to CLL. To find out potential determinants of reduced apoptotic priming in RS, we analyzed the expression and spatial localization of pro-apoptotic members, and performed a morphometric assessment of the mitochondrial cristae. While the pro-apoptotic members Bak, Bax, Bim, and Bid were expressed at comparable levels in CLL and RS cases, they less frequently colocalized with mitochondria in RS cells, as assessed by immunofluorescence microscopy. Moreover, the pro-apoptotic sensitizer Puma was recurrently downmodulated at the protein level in RS. Transmission electron microscopy highlighted that RS mitochondria had reduced cristae width as compared to CLL, suggesting that cristae remodeling might also contribute to sequester cyt c within the intermembrane space during disease transformation. Consistently, the mitochondrial protein ClpB, which plays a role in cristae tightening, was upregulated in RS cases.
In sum, RS cells evolve multiple mechanisms to lower the apoptotic priming and hijack the anti-apoptotic dependencies away from Bcl-2. Further investigations are underway to uncover how such mechanisms could be dismantled for therapeutic purpose.
Disclosures
Vaisitti:Astra Zeneca: Research Funding; Solve Therapeutics: Research Funding. Deaglio:Solve Therapeutics: Research Funding; Astra Zeneca: Research Funding. Ferrarini:Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal